Pharmacovigilance also known as drug safety is defined as "the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug related problems". It plays a key role in ensuring patients receive safe and appropriate drugs. (Lakshmi, Aashritha, Teja, & Sridhar, 2016). Pharmaceutical companies and regulatory authorities utilize signal detection as a tool within pharmacovigilance to ensure the patient’s safety by actively analyzing data for increased risk in medical drugs. Pharmacovigilance signal detection is the process of identifying and investigating potential signals of adverse drug reaction. Signals can be identified through a variety of sources, including the pharmacovigilance database, medical literature, social media, and spontaneous reports from healthcare professionals and patients. However, due to its sensitive nature and regulatory requirements, pharmacovigilance data is not always accessible or reliable. (Kumar & Khan, 2015). As the pharmacovigilance industry continues to grow, artificial intelligence is being implemented into the field to assist in data capturing and processing, forecasting, data security, and signal detection.
“Artificial intelligence (AI) is the ability of a digital computer to perform tasks commonly associated with intelligent beings “(Copeland, 2023). According to Copeland, machines can be built with characteristics of humans, such as the ability to reason, discover meaning, generalize, or learn from past experience. Machine learning is where new capabilities are incorporated into machines to ‘learn’ without explicit programming (Das S, 2015) and create algorithms to accomplish a task while learning from its successes and failures (Hashimoto DA, 2018). The integration of AI into the healthcare system is changing the role of healthcare providers and creating new potential to improve patient safety outcomes (Macrae C, 2019) and quality of care. In pharmacovigilance, the use of artificial intelligence is becoming widely accepted and demanded in various areas including safety operations, identification of target populations, and signal management.

Artificial intelligence is a rapidly growing field of technology that has a lot of potential in pharmacovigilance. As Individual case safety report volume is increasing year to year, 90% of adverse events (AEs) go unreported and improved technologies must be implemented necessary to maintain patient safety. AI offers considerable advantages over human experts when it comes to data integrity, data mining, and regulatory compliance by improving data quality, providing real-time data insights during processing, and enabling faster and more proactive signal detection (Traverso, Aug 2020). It helps in decision-making in a complex situation, (Salas1 M, 2022). For example, with AI performing data mining, it can collect and assess large amounts of data to assist in the process of risk assessment, which leads to improved patient safety.
There are multiple applications which utilize artificial intelligence in pharmacovigilance. Some practices of AI include identification of ADEs (adverse drug events) and ADRs (adverse drug reactions), the performance of drug surveillance, classification of free text within safety reports, (Bates DW, 2021) extraction of drug–drug interactions, identification of populations at high risk of experiencing drug toxicity, prediction of drug side effects, simulation of clinical trials, and signal detection.
Signal detection is an essential part of drug use and safety surveillance. (R H Meyboom 1, 1997). Signal detection can be “qualitative” built on case-by-case assessment of individual case safety reports (ICSRs) or “quantitative” via adopting machine learning or mining approaches using databases of real-world data. Quantitatively/qualitatively detected signals necessitate further validation and confirmation by clinical judgment. A signal can be new safety information or a new aspect to an already known adverse drug reaction which suggests a causally related association to a medication(s) of which warrants further investigation to accept or refute. These potential medicine safety issues can be detected from different data sources such as ICSRs, spontaneous reporting systems, scientific literature, biomedical databases, and electronic health records.
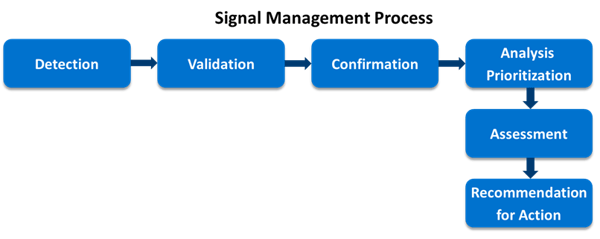
Signal detection is a cornerstone of the drug development process ensuring patient safety. With the early detection of potential problems, this not only improves patient safety but also helps to reduce economic burden associated with escalating cost of medications and unwanted adverse effects associated with it. Newly identified signals can be potentially helpful in mitigating patient suffering in the future by building evidence-based research outcomes. Although, to detect the unknown and unexpected signals as soon as possible from the market is the major challenge in pharmacovigilance. (Ritu Rani, 2019).
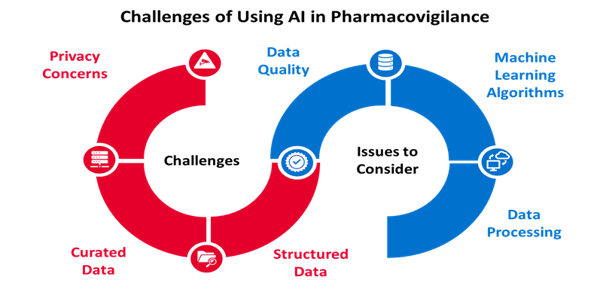
Pharmacovigilance is a critical and essential function in healthcare. However, the use of artificial intelligence is still relatively new and developing within the field. One of the main challenges in adopting to AI is availability of structured and curated data for training the software to identify potential drug safety issues. Additionally, there are privacy concerns with using AI for pharmacovigilance, as data could potentially be used for other purposes without consent from individuals involved. Pharmacovigilance systems can be fragmented with missing information, making machine learning difficult and inconsistent. As a result, many companies have taken a phased approach to implementing AI into their business model. One way to begin utilizing this method is for pharmacovigilance teams to insert AI in the safety database. There the AI can assist in data collection and case comparison ensuring data consistency. This step-like implementation will allow a pharmacovigilance associate team member to perform a quality check of the machine by manually comparing the data output provided by AI and the data source received. Once the team has initiated artificial intelligence into this process, they can begin to insert AI into more complicated processes like automatically generating narratives, full case processing, and analytics of safety data for signal detection. Artificial intelligence is the future of pharmacovigilance with managing large amounts of data and performing complete, accurate data analysis.
Author:
Bawneet Narang
Pharmacovigilance Associate – Linical
References:
- Bates, D.W., Levine, D., Syrowatka, A., Kuznetsova, M., Craig, K.J.T., Rui, A., et al (2021). The potential of artificial intelligence to improve patient safety: a scoping review. NPJ Digital Medicine, 4, 54.
- Macrae, C. (2019). Governing the safety of artificial intelligence in healthcare. BMJ Quality Safety, 28(6), 495–498.
- Copeland, B. (2023, APR 25). Artificial Intelligence. Retrieved APR 25, 2023, from Britannica: https://www.britannica.com/technology/artificial-intelligence.
- Das, S., Dey, A., Pal, A., Roy, N. (2015). Applications of artificial intelligence in machine learning: review and prospect. International Journal of Computer Applications, 115, 31-41.
- Hashimoto, D.A., Rosman, G., Rus, D., Meireles, O.R (2018). Artificial intelligence in surgery: promises and perils. Annals of Surgery, 268(1), 70–76.
- Kumar, A., Khan, H. (2015). Signal Detection and their Assessment in Pharmacovigilance. Open Pharmaceutical Sciences Journal, 2, 66-73.
- Lakshmi, A., Aashritha, M., Teja, K., Sridhar, R. (2016). A Review On Pharmacovigilance and Its Importance. World Journal of pharmacy and pharmaceutical sciences, 6(1), 300-310.
- Meyboom, R.H., Egberts, A.C., Edwards, I.R., Hekster, Y.A., Koning, F.H. de., Gribnau, F.W. (1997). Principles of signal detection in pharmacovigilance. National Library of Medicine, 16(6), 355-365.
- Rani, R., Chand, S., Devi, M., Singh, A., Kumar, D. (2019). A Review on Signal in Pharmacovigilance. Asian Journal of Pharmaceutical Research and Development, 7(6), 80-84.
- Salas, M., Petracek, J., Yalamanchili, P., Aimer, O., Kasthuril, D., Dhingra, S., et al (2022). The Use of Artifcial Intelligence in Pharmacovigilance: A Systematic Review of the Literature. Pharmaceutical Medicine, 36 (5), 295-306.
- Traverso, K., Markey, J. (Aug 2020). The Untapped Potential of AI & Automation in Pharmacovigilance. Pharmaceutical Engineering. Retrieved APR 26, 2023: https://ispe.org/pharmaceutical-engineering/july-august-2020/untapped-potential-ai-automation-pharmacovigilance
.jpg)