
Quality Assurance
At Linical, quality is about more than just compliance for us. Our attentive team is focused on the well-being and safety of patients as well as the integrity of your clinical trial data.
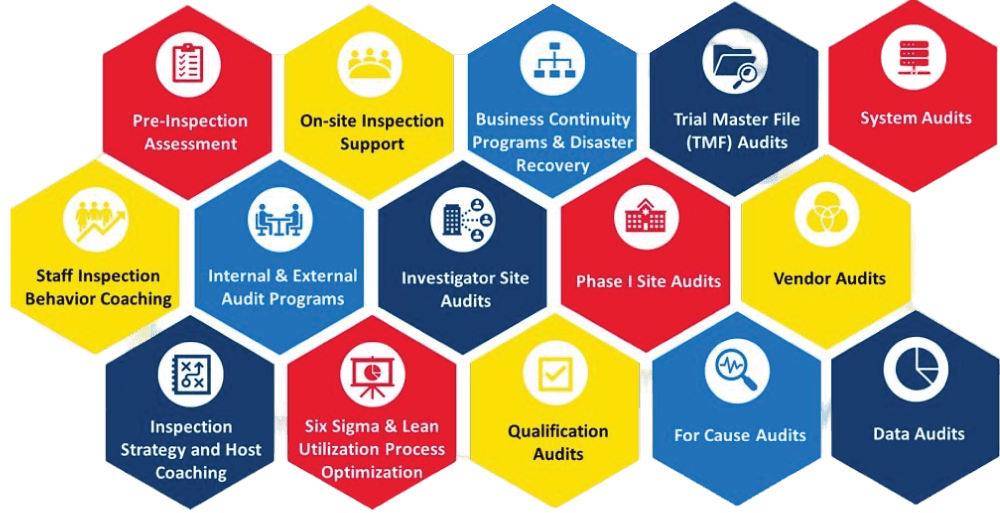
Quality Assurance Services
Linical has QA professionals with extensive experience and knowledge of GCPs to support our clients in acting in the best interest of the study, optimizing their processes and quality systems, and complying with all applicable rules and regulations.
Our comprehensive quality assurance services include:
Process
Quality Assurance and Compliance for Clinical Research
Explore
- 21 CFR (Code of Federal Regulations) Parts 11, 50, 54, 312 and 820
- ICH E6; Guidelines for Good Clinical Practice
- EU Directives
- Policies, SOPs, working practice documents (WPDs), forms, templates and industry standard validated computer system applications
- Standardized and controlled global Quality System Documents (QSDs)
- Documented QSD and project-specific training for all staff via a best-in-class learning management system
- Facilitate Client audits
- Support for GCP questions and compliance issues
- Available to project team as needed
- Resource to clients regarding Quality Assurance needs
- Perform vendor, site, CSR, and TMF audits
- Facilitate and manage regulatory inspections
Quality Planning for Project Success
Linical is a strong advocate for the development and use of Quality Plans to support Sponsor-CRO projects and relationships. Early development and use of Key Quality Indicators (KQIs) are critically important to standard governance. Linical’s partnership in supporting Sponsor’s study oversight responsibilities, includes consultation in the development of appropriate and meaningful Quality Tolerance Limits (QTLs), where desired. Monitoring and acting upon these limits within the governance model provides Sponsors with the visibility and control to meet their responsibilities.
The Highest Standard of Quality for Your Clinical Trial
Why Linical?
The clinical development journey can be daunting and often leads to failure. With so much riding on your compound, you deserve the best chance at achieving your goals and positively impacting patients across the globe.
As a global, award-winning CRO, we can provide the strategy and support you need to position your clinical trial for success. We have an impressive track record of exceeding our enrollment goals and maintaining nearly a 90% client retention rate.
With our collaborative approach and commitment to quality, Linical expertly guides you through each step of the process, from early-phase research to large-scale global studies. With Linical, you can overcome obstacles, expedite timelines, save valuable money, and achieve your goals without compromising quality.
Successful clinical trials start with Linical.
Don’t let the complex clinical development journey hold you back. With Linical, you can overcome obstacles, save valuable time and money, and reach your goals.
01 Request a proposal
We start by listening to your needs and understanding your goals to ensure we’re the right CRO for you.
02 Get a plan for success
We propose solutions that proactively tackle obstacles, optimize your trial design, and position you for success every step of the way.
03 Execute with confidence
We’ll guide you through each phase of the process, offering personalized support and a full range of services to help you achieve a successful trial. We are not a “one size fits all” CRO.
.webp?width=518&height=780&name=cta-img%20(2).webp)